If you have aortic stenosis, you may be eligible for the
ENVISION Clinical Trial
If you have aortic stenosis, you may be eligible for the
ENVISION Clinical Trial

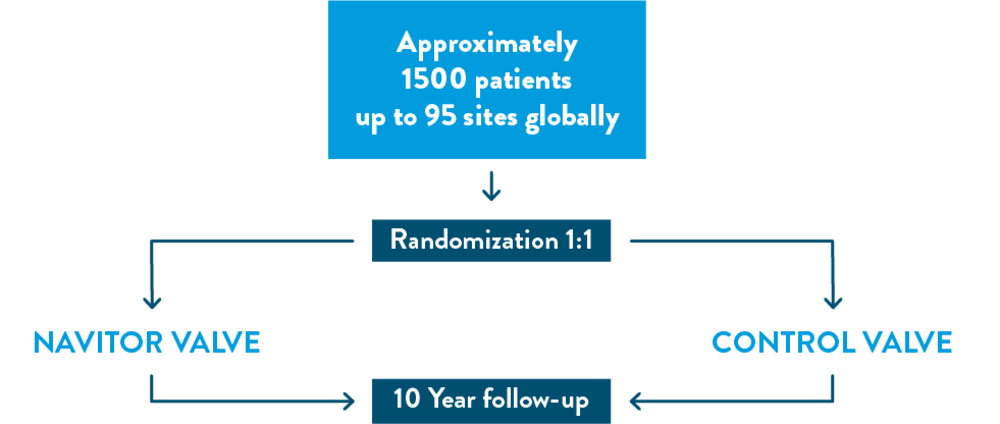
The ENVISION Clinical Trial is studying the Navitor™ Transcatheter Aortic Valve Implantation (TAVI) System in patients with severe, symptomatic aortic stenosis who are deemed low or intermediate surgical risk.
Approximately 1,500 patients will be studied at up to 80 medical centers in the United States and up to 15 medical centers outside of the United States. A qualified team of doctors will monitor the patients included in the ENVISION Clinical Trial.
Clinical trial participants will play an important role in helping doctors evaluate the use of the Navitor TAVI System for patients with severe aortic stenosis.
NCT05932615 ClinicalTrials.gov
Find a clinical trial site near me
WHAT IS
AORTIC STENOSIS?
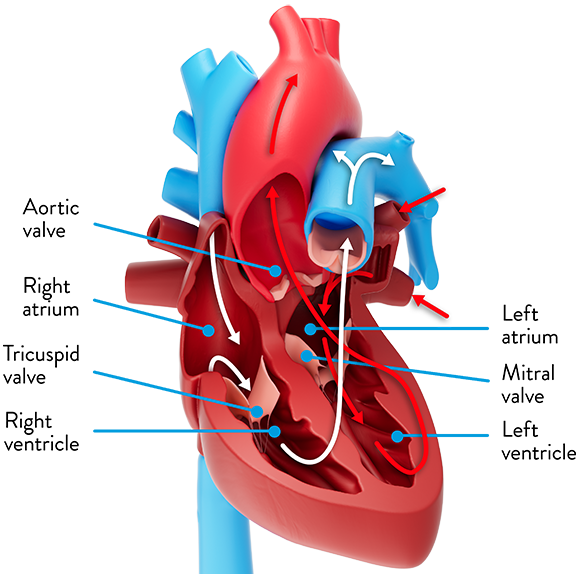
On the left side of the heart, blood flows through the left ventricle into the aorta through the aortic valve. Severe aortic stenosis is a condition in which the aortic valve leaflets become thick or stiff, reducing their ability to fully open and close. This narrowing of the valve makes your heart muscle work harder to deliver oxygen-rich blood to the brain and the rest of your body.
Aortic stenosis is one of the most common valve diseases and usually develops later in life.
Causes include:
- Buildup of calcium on the valve
- Infection of the heart
- Birth defects
- Rheumatic fever
- Radiation therapy

WHAT ARE
THE SYMPTOMS?
The symptoms of aortic stenosis are commonly misunderstood by patients as “normal” signs of aging. Symptoms of aortic stenosis include, but are not limited to:

Trouble breathing

Chest pain

Dizziness, lightheadedness, or even fainting

Fatigue with
normal activities

Difficulty walking short distances

Rapid, fluttering heartbeat

Difficulty sleeping

Swollen ankles
or feet
Treatment options for
severe aortic stenosis
Based on your level of risk, the outcomes from your diagnostic tests and lifestyle preference, your doctor will recommend the best treatment option for you. There are various treatment options for severe aortic stenosis including medication, balloon aortic valvuloplasty, surgical aortic valve replacement or transcatheter aortic valve implantation.
Surgical Aortic Valve
Replacement (SAVR)
Aortic valve replacement can be performed using several surgical approaches to open-heart surgery, including traditional sternotomy (with an incision over the chest and sternum), minimally invasive surgery that does not involve opening the chest, and less invasive robotic procedures.
Transcatheter Aortic Valve
Implantation (TAVI)
Transcatheter aortic valve implantation provides an alternative, minimally invasive treatment option for people living with severe aortic stenosis.
What will happen
during the procedure?

STEP 1
Your doctor will determine the best approach for replacing your valve, but the most common approach is the transfemoral approach, described here. A small cut is made in a large artery in your groin and a sheath (access tube) is inserted within your artery. The doctors view the movement of the sheath using special imaging equipment, which guides them to know when the sheath is in the correct position.

STEP 2
Once the sheath is in position, a guiding wire and balloon catheter (thin, flexible tube) with a balloon is delivered through the sheath and placed within your aortic valve. The balloon is inflated to open up your aortic valve as much as possible so that the Navitor™ Valve can be placed inside of it. The balloon catheter is then removed.

STEP 3
The Navitor Valve is delivered with a catheter through the sheath and placed within your opened aortic valve. Your doctor will deploy the valve into the correct positioning using special visualization equipment to ensure the valve is positioned accurately within your heart.
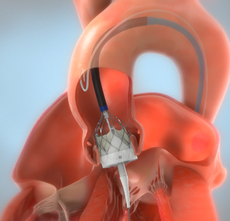
STEP 4
Once the valve is deployed and accurately in place, the Navitor Valve will start to function immediately.
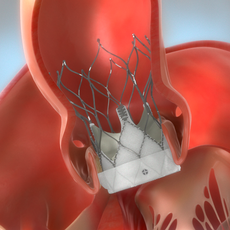
STEP 5
The sheath and catheter are removed from your heart and groin, and the small incision in your groin is closed or sealed. The procedure is now complete.
ARE YOU A CANDIDATE FOR
THE ENVISION CLINICAL TRIAL?
Who can participate in the ENVISION Clinical Trial?
Both men and women can participate. You may qualify if:
- You have been diagnosed with severe, symptomatic aortic stenosis.
- It has been determined by your doctor that you are low or intermediate surgical risk and a transcatheter valve is appropriate for you.
What is involved if I choose to participate?
Before you are enrolled, you will be screened to make sure you are a good candidate for the clinical trial. Your screening may include:
- An in-person visit with your heart team, which includes the informed consent process.
- An echocardiogram (ECHO) to determine if you have severe aortic stenosis.
- A computerized tomography (CT) scan to determine the appropriate valve size.
- A review of your medical records.
- Blood tests
You can still see your personal cardiologist for regular checkups and care. In addition, you will be monitored by regular follow-up exams.
What happens if I pass the screening process?
- You will be assessed in person at the treatment center and then registered for the clinical trial.
- You will be randomized to either the Navitor™ TAVI System or an alternative, commercially available TAVI system.
- You will have your TAVI procedure.
- You will also have in-person or phone call follow-up exams with healthcare providers at 30 days and annually through 10 years.
- You will have the option to have some of your in-person follow-up visits in the comfort of your own home.
The ENVISION
patient journey
Navitor™ Transcatheter Aortic Valve Implantation (TAVI) System
The Navitor TAVI System is an FDA approved device to treat patients with symptomatic, severe aortic stenosis who are at high or extreme surgical risk. The ENVISION Clinical Trial aims to expand the approved use of the Navitor TAVI System to treat patients with symptomatic, severe aortic stenosis who are at intermediate or low surgical risk. In this patient population, the Navitor TAVI System is limited by U.S. law to investigational use only and not for commercial use.
- Importance of clinical research and the ENVISION Clinical Trial
- The Navitor TAVI System is FDA approved, but currently only for patients at high and extreme surgical risk. The ENVISION trial is evaluating the use of the Navitor TAVI System in patients with symptomatic, severe aortic stenosis who are at intermediate or low surgical risk. The results of this trial may expand availability of the Navitor TAVI System to a new group of patients who are candidates for TAVI.
- This trial allows doctors to compare treatments (Navitor TAVI System and commercially available TAVI systems) for safety and effectiveness.
- May help future patients with similar health conditions receive the best treatment.
- Importance of diversity in clinical trials
Diversity in clinical trials is critical to represent the real-world population. If applicable (women, minorities), your participation is especially impactful. For more information on the importance of diversity in clinical trials, please visit Clinical Trial Diversity is Key To Future Innovation | Abbott U.S.
FREQUENTLY
ASKED QUESTIONS
- What will happen if I decide I am interested to take part in this trial?
This research study includes a screening/qualification phase to determine if you are a good candidate for the study. Your trial doctor or other study personnel will ask you medically related questions. You will undergo testing as part of standard of care for undergoing a transcatheter aortic valve implantation (TAVI) procedure. Additional tests may be conducted to determine if you qualify for the study. Once all screening tests have been completed, your data will be deidentified and sent to an independent committee of experienced doctors who will confirm if you qualify to take part in the study. If the committee decides that you do not qualify for the study, your participation will end.
If you do qualify, and you decide to take part in this research study, you will return to the clinic for annual follow-up visits over the 10-year follow-up period. You are expected to complete all required follow-up visits. Your local clinical research team will partner with you to schedule follow-up visits alongside your standard of care follow-up visits with your medical care team.
- Do I have a choice in what TAVI valve I receive to treat my aortic stenosis?
If qualified for the study, you will not have a choice in what TAVI valve you receive as the ENVISION Clinical Trial is a randomized controlled study.
Randomization is the process by which you will be randomly (by chance) assigned to either of two groups (control group or treatment group). Like in a coin toss, you will have an equal chance of being assigned to the control group or the treatment group. A computer program will decide to which group you are assigned. The control group will receive an FDA approved TAVI system, and the treatment group will receive the Navitor TAVI system by Abbott. The Navitor TAVI system is already FDA approved for treating your valve condition in patients at high and extreme risk for open-heart surgery. The ENVISION Clinical Trial aims to expand the approved use of the Navitor TAVI system to patients at intermediate and low risk of open-heart surgery.
You as well as your doctor who performs the TAVI procedure will be aware of your group assignment. Irrespective of the group you are assigned, you will return for visits with your trial doctor following the same schedule.
- What happens if I am randomized to the control group?
If you are assigned to the control group, you will undergo a TAVI procedure using a valve that is already FDA approved for patients at intermediate and low risk of open-heart surgery. Your trial doctor will select the appropriate valve for you.
- What happens if I am randomized to the treatment group?
If you are assigned to the treatment group, you will undergo a TAVI procedure using the Navitor TAVI system. The Navitor TAVI system is already FDA approved for treating your valve condition in patients at high and extreme risk for open-heart surgery. The ENVISION Clinical Trial aims to expand the approved use of the Navitor TAVI system to patients at intermediate and low risk of open-heart surgery.
- Where can I learn more about patients who have been treated with the Navitor TAVI system by Abbott?
You can learn more about the Navitor TAVI system by Abbott at Aortic Valve Replacement
- Where can I learn more about the ENVISION Clinical Trial?
You can learn more about participating in a clinical trial and the ENVISION Clinical Trial at Structural Heart Clinical Trials.
- What other choices do I have?
You will receive available treatment for symptomatic, severe native aortic stenosis whether or not you take part in this trial. If you choose not to take part in this trial, your doctor will treat you according to standard medical care. Your doctor will explain in more detail the various alternative treatment options available to you and the risks and benefits of these alternatives. These treatment options may include:
- Implantation of a commercially available TAVI valve for patients at intermediate and low risk of open-heart surgery
- Standard open-heart surgery by referral to a heart surgeon. For more information, visit Aortic Valve Replacement
Your future care and your relationship with your doctor will not be affected if you decide not to be in this trial.
- Why should I participate in this clinical trial?
For more information on why to participate in a clinical study, visit Structural Heart's clinical trials page
MAT-2310081 v3.0 | Item approved for U.S. use only.