BUILT FOR BETTER LIFE
We partner with some of the world's best specialists and care teams that we know, to repair the damage caused by structural heart diseases and help rebuild healthier hearts—through better therapies, better evidence, and better experience. It’s that simple.
Partnering in excellence
We partner with healthcare providers to deliver unwavering training and support, lending our clinical insights into patient selection, echocardiography and diagnostics, device therapies, and procedural details. All to help physicians deliver the full potential of every Abbott technology.
OUR PROMISE
Better therapies
We’re leading the way for structural heart therapies and challenging current treatment options to change the course of patient care.1
- Read more
Our comprehensive portfolio is designed to be as minimally invasive as possible and to treat a broad range of patients with structural heart diseases, from infants to elderly patients. We’ve set a new standard of care as we relentlessly focus on delivering better solutions to more patients.


Better evidence
Our clinical trials provide proof that inspires confidence for physicians and patients.1
- Read more
With successful clinical results from tens of thousands of patients across the structural heart portfolio and over 40 active clinical trials, we continue to go above and beyond as we grow the body of evidence for our structural heart devices. Our legacy of leadership and expertise back us as we build better therapies for the growing number of structural heart patients.
Better experience
We’re dedicated to helping care teams deliver the full potential of every structural heart procedure, from procedural training and imaging expertise to proctorship support and beyond.1
- Read more
Our patient-first focus means we prioritize maximizing benefits and minimizing risk for all patients, so physicians can help them reclaim their quality of life. We build deep, trusted partnerships with healthcare providers to ensure that everyone and everything beats as one.

The aging global population has intensified the burden of structural heart disease
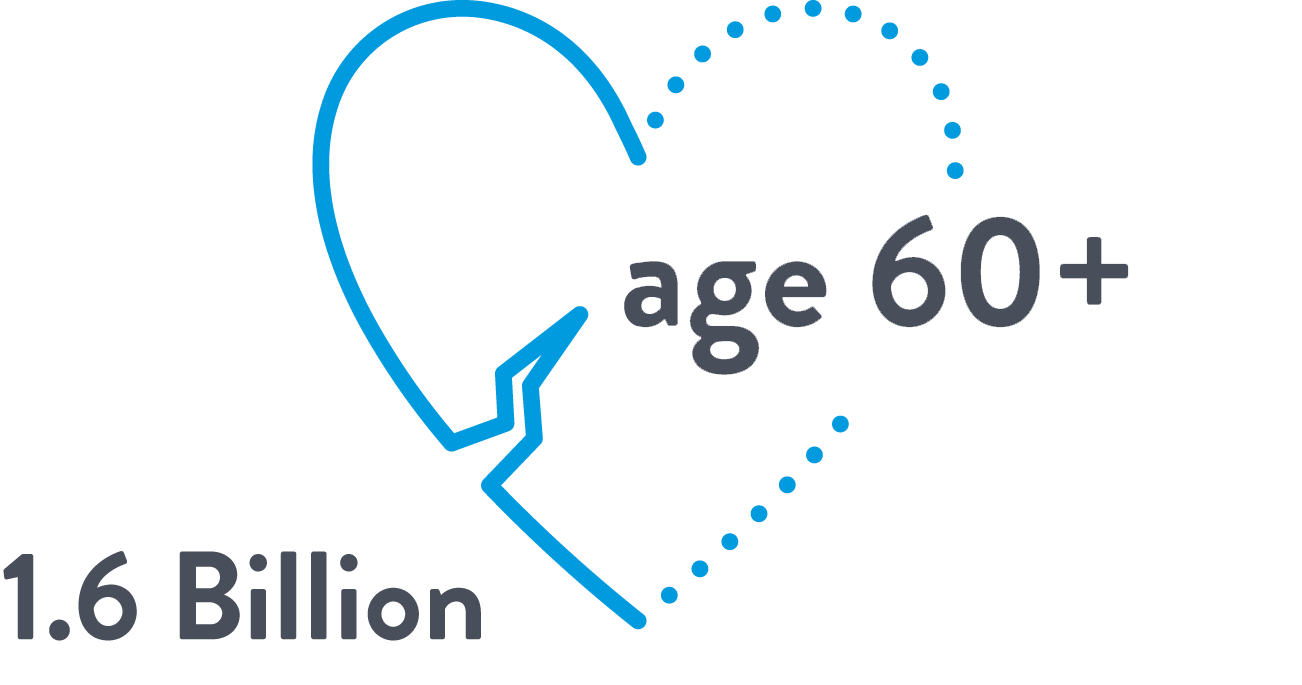
1.6 BILLION
PEOPLE AGED 60 OR OLDER
BY 20302
1 in 10 PEOPLE
OVER 65 YEARS OLD HAVE
MODERATE OR
SEVERE VALVE DISEASE3
#1 CAUSE OF DEATH
HEART DISEASE REMAINS
NO. 1 CAUSE OF DEATH4
OUR SUPPORT
Partnering in excellence
We partner with healthcare providers to deliver unwavering training and support, lending our clinical insights into patient selection, echocardiography and diagnostics, device therapies, and procedural details. All to help physicians deliver the full potential of every Abbott technology.

- Procedural training
Procedural training
Our team of clinical experts are with your staff for every training procedure, committed to consistent guidance every step of the way.
- Imaging expertise
Imaging expertise
As transcatheter valve therapies advance, so do imaging technologies—and with them, the need for a trusted partner. Our proctors are highly trained to support your team’s imaging efforts and have the deep procedural experience to help troubleshoot any scenario.
- Ongoing support
Ongoing support
From the robust initial training new centers undergo, to proctoring and case monitoring, and supplementary training offerings, our support enables your continued growth in efficiency and outcomes from day one and beyond.
Little heroes need our support
See how Abbott's Little Heroes initiative provides life-changing technologies and support for children that do not have access to healthcare.
MAT-2503485 v1.0 | Item approved for OUS use only.