TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR CLINICAL DATA
TriClip™ Transcatheter Edge-to-Edge repair offers a minimally invasive treatment option for patients with severe symptomatic tricuspid regurgitation (TR).
TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR CLINICAL DATA
TriClip™ Transcatheter Edge-to-Edge repair offers a minimally invasive treatment option for patients with severe symptomatic tricuspid regurgitation (TR).
INSPIRED BY PATIENTS. MADE POSSIBLE BY YOU.
TriClip TEER has shown meaningful outcomes in both real-world and randomized clinical data. The bRIGHT study and TRILUMINATE™ Pivotal trial demonstrated the safety and effectiveness of TriClip TEER in the treatment of severe tricuspid regurgitation (TR).2-6
Exceptional safety.*2,10
Life-changing impact.2,9,10
The TRILUMINATE™ Pivotal Trial demonstrated that TriClip TEER therapy was superior to medical therapy alone in improving quality of life and reducing tricuspid regurgitation.
Remarkable and sustained TR reduction2
TR grade (Core Lab)
- Exceptional safety2,10 at 30 days
98.3%
FREEDOM
FROM MAEs0.6%
NEW PACEMAKER
IMPLANTATION0%
DEVICE THROMBUS
99.4%
SURVIVAL
0%
NONELECTIVE CV
SURGERY FROM
DEVICE-RELATED AE0%
DEVICE EMBOLIZATION
- Significant improvement in health-related quality of life2
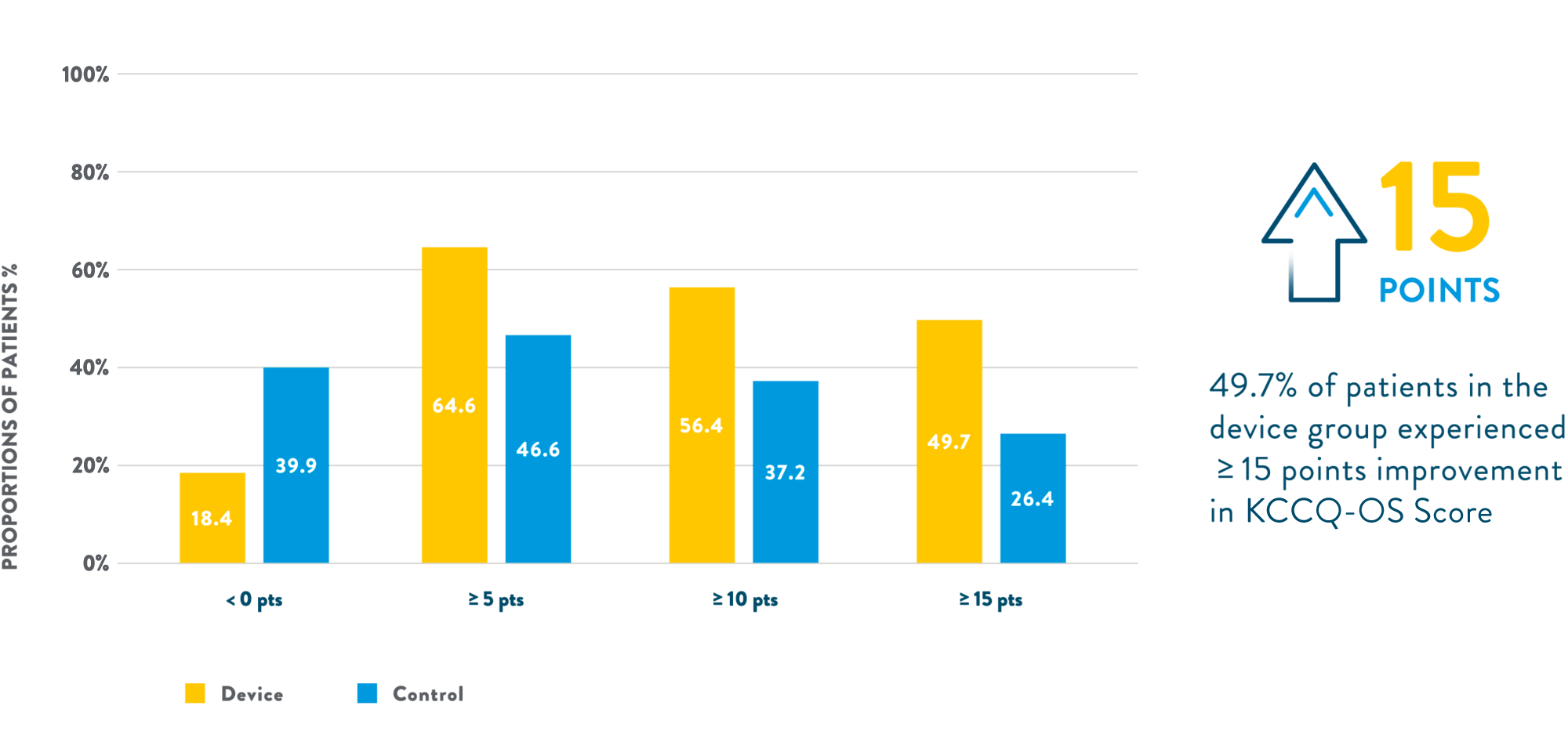
- Baseline population characteristics2
71%
OF PATIENTS HAD MASSIVE
OR TORRENTIAL TR4.4±0.7cm
TRICUSPID VALVE
ANNULUS DIAMETER>35%
OF PATIENTS WITH PRIOR
MITRAL OR AORTIC VALVULAR INTERVENTION15%
OF PATIENTS HAD A CRT, CRT-D, ICD,
OR PERMANENT PACEMAKER*At 30 days
TRILUMINATE PIVOTAL TRIAL DATA CARD
Get the latest data from the TRILUMINATE Pivotal Trial in a one-pager PDF format.
Proven across a broad
range of anatomies7,8
The bRIGHT Study demonstrated that TriClip™ TEER significantly reduced TR across a broad range of anatomies - in a safe and effective procedure - resulting in durable and meaningful clinical outcomes.
Designed to maximize TR reduction7
TR grade (Core Lab)
- Successfully treated a broad range of anatomies8
- Proven durability backed by clinical data
NYHA Functional Class7
KCCQ - OS Score12
- TriClip TEER demonstrated procedural success and an exceptional safety profile5
HIGH PROCEDURAL
SUCCESS99.0%
Implant
sucess rateSHORT
DEVICE TIME
76±39
Minutes
HIGH SAFETY
PROFILE
97.5%
Freedom
from MAEs
at 30 days
99.0% survival at 30 days0.2% TV reintervention
0.0% embolization
bRIGHT STUDY DATA CARD
Get the latest data from the bRIGHT Study in a one-pager PDF format.
Proven safety and effectiveness
The TRILUMINATE™ Trial demonstrated that TriClip TEER safely and effectively reduces TR and HF hospitalizations.
93%
SURVIVAL
AT 1 YR3
100%
IMPLANT
SUCCESS4
91%
ACUTE PROCEDURAL
SUCCESS RATE4
0%
STROKE4
0%
CONVERSION TO
SURGERY4
- Significant and durable TR reduction11
Durable reduction in tricuspid regurgitation (TR)
- Significant improvement in function and quality of life11
Durable improvements in NYHA
≥10-point KCCQ-OS score improvement was observed in 50% of subjects at three years.11 - Significant reduction in hospitalizations4
Reduced hospitalization rate
TRILUMINATE TRIAL DATA CARD
Get the latest data from the TRILUMINATE Trial in a one-pager PDF format.
MAT-2006556 v11.0 | Item approved for OUS use only.

