CONGENITAL HEART DEFECTS
Congenital heart defects are the most common type of birth defect. Defects that involve the wall or vessels of the heart include atrial septal defect (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). In certain situations, guidelines recommend surgery or transcatheter device closure to repair the defect and prevent complications.1
ATRIAL SEPTAL DEFECT (ASD)
Incidence, morbidity, and mortality with atrial septal defect
30%
Among congenital heart disease diagnosed in adulthood, 30%-40% are ASD2,3
61%
In pediatric patients, 61% of ASDs present as complex lesions4
50%
Adults with unrepaired secundum defect have reduced exercise capacity of 50%-60%2
4.5%
Untreated large secundum ASD annual mortality rates2:
For patients in their 40s: 4.5%
For patients in their 60s: 7.5%
One study examined long-term mortality of adult ASD patients with and without closure of the defect. The findings reveal that mortality overall was higher among ASD patients without closure compared to patients who had ASD closure, and cardiac deaths were significantly higher (p < 0.01). For instance, heart failure accounted for 12% of deaths in non-closure patients vs 2.5% among patients who had ASD closure.5
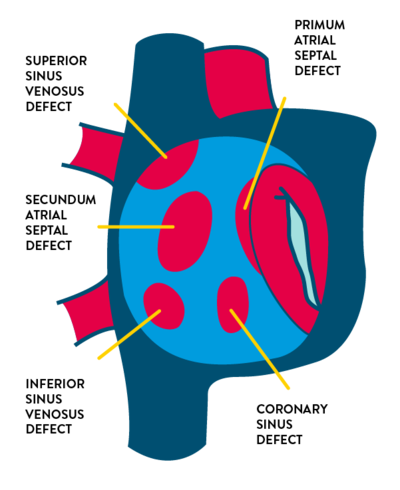
- Types of atrial septal defect
- Secundum ASD (75% of cases) in the region of the fossa ovalis
- Primum ASD (15% to 20%) located inferiorly near the crux of the heart
- Sinus venosus ASD (5% to 10%) located near:
- Superior vena cava entry
- Inferior vena cava entry
- Coronary sinus defect (less than 1%) which causes shunting through the coronary sinus ostium
About 65%-70% of patients with a secundum defect are female.2
- Causes of atrial septal defect
Most such defects have no identifiable cause, but certain genetic abnormalities have been linked to ASDs. The risk of a secundum defect in particular is increased in families with a history of congenital heart disease—particularly when ASD has also been diagnosed in a sibling.2
ASDs have been associated with maternal characteristics and behaviors such as2:
- Fetal alcohol syndrome
- Cigarette smoking, particularly in the first trimester
- Advanced maternal age (≥ 35)
- Certain antidepressant use
- Diabetes
- When symptoms develop
Most children with isolated ASDs are asymptomatic. If patients are untreated, however, symptoms can occur in adulthood.2
Symptoms of atrial septal defect
The consequence of a left-to-right shunt across an ASD is right ventricular volume overload and pulmonary over-circulation. Patients who have large atrial shunts can experience symptoms related to excess pulmonary blood flow and right-sided heart failure. In infants, children, and young adults up to approximately age 20, symptoms may include:
- Heart murmur (followed up with echocardiogram)
- Frequent respiratory infections
- Slow weight gain
In older adults ASDs may present with symptoms of:
- Dyspnea
- Palpitations
- Fatigue and exercise intolerance
- Cyanosis
- Atrial arrhythmias—atrial flutter, atrial fibrillation, and sick sinus syndrome
- Peripheral edema
- Cardiomegaly on routine chest x-ray
- More audible murmur during pregnancy
- Paradoxical embolism

- Symptoms in patients with small ASD
Patients who have small defects (< 10 mm) have minimal to no enlargement of the right heart structures, and these patients can remain asymptomatic into their 40s or 50s. But even these patients may develop symptoms with increasing age. Symptoms appear over time for several reasons—in 18% of cases because of an increase in the size of the defect (although this is more likely to occur in those who initially have larger defects).2 Increased shunting can also be a factor, caused by a decrease in left ventricular (LV) compliance as the patient develops coronary artery disease, hypertension, or acquired valvular disease.
To avoid late diagnosis: awareness of gradual onset of symptoms
Because many patients experience the gradual onset of symptoms—and may exhibit only subtle physical findings—late diagnosis can occur in patients with ASDs. Proper attention to symptom presentation can preclude late diagnosis, which puts patient at higher risk for arrhythmias, pulmonary arterial hypertension, LV systolic dysfunction, and paradoxical embolism.
Diagnostic evaluation
Transthoracic echocardiography (TTE) is the primary imaging modality for an ASD. Clinicians should visualize the entire atrial septum, from the orifice of the superior vena cava to the orifice of the inferior vena cava using multiple views. This is important given that poor quality transthoracic images can produce false-negative diagnoses, which can be relatively common.
Treatment for Atrial Septal Defects
Percutaneous device or surgical closure are the mainstays of therapy in patients with hemodynamic or clinical consequences of the defect. However, severe pulmonary arterial hypertension (PAH) is a contraindication to closure.1 Percutaneous closure is the treatment of choice in the majority of cases of secundum ASDs.6
VENTRICULAR SEPTAL DEFECTS (VSD)
A VSD is common in both children and adults second only to bicuspid aortic valves as the most common congenital heart defect. Spontaneous closure occurs most often in muscular defects; it also occurs in 35%-40% of patients with perimembranous defects. Spontaneous closure frequently occurs in children, usually by age 2 years; it is uncommon after age 4.7
Data show that spontaneous closure is decreased in patients who have VSD in addition to patent ductus arteriosus (PDA), likely because PDA further increases left-to-right shunt and leads to more severe hemodynamic effects.8
- Types of ventricular septal defects
There are 4 types of VSDs:
- Perimembranous VSDs—the most common, composing 80% of VSDs—are located in the membranous septum, adjacent to the septal leaflet of the tricuspid valve. On the left ventricular (LV) side, the defect is adjacent to the aortic valve.
- Muscular VSDs can be located centrally, at the apex, or at the margin of the septum and right ventricular free wall. Although muscular VSDs account for up to 20% of VSDs in infants, spontaneous closure reduces their incidence in adults.
- Conal or subpulmonary VSDs, found just beneath the pulmonary and aortic valves, occur in about 6% of defects in non-Asian populations and up to 33% in Asian populations. Spontaneous closure is uncommon.
- Inlet VSDs, which occur in the lower right ventricle and adjacent to the tricuspid valve, typically occur in patients with Down syndrome.
- Cardiovascular effects of ventricular septal defects
A left-to-right shunt resulting from the VSD can result in:
- Increased LV volume load
- Excessive pulmonary blood flow
- Reduced systemic cardiac output
- Elevated pulmonary artery pressures
- Complications from ventricular septal defects7
Even though patients with small perimembranous VSDs tend to have good outcomes, that category of VSD is associated with an increased risk of:
- Aortic valve prolapse
- Malignant ventricular arrhythmias
Eisenmenger complex is the most severe complication of a large VSD. Irreversible pulmonary hypertension develops, resulting in the left-to-right shunt reversing to become a right-to-left shunt. Patients with Eisenmenger syndrome require therapy.
- Contributing factors to ventricular septal defects
Among the contributing factors are:
- A congenital cardiovascular defect in a parent or sibling
- Maternal diabetes
- Maternal alcohol consumption (for muscular VSD)
VSDs are the most common lesion in many chromosomal syndromes, including trisomy 13, trisomy 18, trisomy 21, and other more rare syndromes. However, for more than 95% of patients with VSDs, there is no association with a chromosomal abnormality.7
- Symptoms correspond to VSD size
Defect size as noted below is presented as a percentage of the aortic annulus diameter.
A small VSD of ≤ 25% has small left-to-right shunts and no LV volume overload. These VSDs usually have a very good prognosis but the defect can:
- Present as a systolic murmur
- Put the patient at risk for infective endocarditis
- For perimembranous VSDs, present an increased risk for aortic cusp prolapse
A moderate VSD of > 25% but < 75% has moderate LV volume overload, and mild or no PAH. Patients may present with:
- No symptoms
- Symptoms of mild congestive heart failure
A large VSD of ≥ 75% has a moderate to large shunt, LV volume overload, and PAH. Infants with a large VSD have symptoms including:
- Dyspnea
- Tachypnea
- Perspiration
- Fatigue while feeding
- Poor weight gain
- Other symptoms in adults
An adult with a VSD may present with:
- A systolic murmur previously thought to be an innocent murmur
- Fever and bacteremia from infective endocarditis
- A new diastolic murmur indicating aortic regurgitation due to aortic valve prolapse
- Cyanosis and exercise intolerance due to the progressive development of pulmonary vascular disease
- Diagnostic evaluation
Doppler echocardiography is the primary means of diagnosing VSDs.
PATENT DUCTUS ARTERIOSUS (PDA)
From week 6 of fetal life until birth, the ductus is responsible for most of the right ventricular outflow. Normally, functional closure of the ductus arteriosus occurs by about 15 hours of life in healthy, full-term infants.
Incidence and mortality with patent ductus arteriosus
- In full-term infants PDA accounts for 5%-10% of all congenital heart disease9
- In preterm infants the incidence of PDA can be up to 60%9
- Among neonates with a birth weight ≤ 2.6 lb, 80% have PDA9
- Among preterm infants presenting with respiratory distress syndrome, about 80% have PDA9
- A PDA that fails to close by 2-3 days after birth may result in infant morbidity and mortality of up to 30%9
- Left untreated, a PDA carries mortality rates of10:
20%
BY AGE 20
42%
BY AGE 45
60%
BY AGE 60
- Morbidities associated with Patent Ductus Arteriosus
Patients with a moderate left-to-right shunt may remain asymptomatic for years. However, historical series have shown that chronic volume overload may ultimately lead to severe complications11:
- Congestive heart failure
- Atrial arrhythmias
- Irreversible hypertensive pulmonary vascular disease
- Endocarditis
- In rare cases: ductus aneurysm or acute aortic dissection
- Congenital comorbidities associated with Patent Ductus Arteriosus
- 47.7% Atrial Septal Defect (ASD)12
- 14.4% Ventricular Septal Defect (VSD)12
- 13.9% Pulmonary Stenosis12
- Patent Ductus Arteriosus classification
The Krichenko classification of PDA, based on angiography, includes 6 types:
The PDA can range from a small hemodynamically insignificant lesion that is not heard on auscultation to one that without intervention is large enough to cause congestive heart failure and pulmonary hypertension.13,11

Patent Ductus Arteriosus

Type A: conical

Type B: window

Type C: tubular

Type D: complex

Type E: elongated

Type F: Fetal
- Mortality rates: why closure is essential
If PDA is left untreated, the mortality rate is 20% by age 20 years, 42% by age 45, and 60% by age 60.11
Complications from an untreated PDA may include heart failure, renal dysfunction, necrotizing enterocolitis, intraventricular hemorrhage, diminished nutrition and growth, and potentially chronic lung disease.
Patients with a large PDA, when untreated, are at risk of developing Eisenmenger Syndrome, in which the usual left-to-right shunting reverses to a right-to-left shunt. At this point the PDA is irreversible, PDA closure is contraindicated, and lung transplantation may be the only option for long-term survival.
Neonatal presentation at physical exam
Assessment can reveal:
- Wide pulse pressure
- Bounding peripheral pulses
- Apnea (in neonates)
- Unexplained metabolic acidosis
- Hypotension and systemic hypoperfusion
Though not often observed in preterm infants, a murmur often obscures the S2. The murmur may be noticeable only during systole, or it may be a crescendo-decrescendo systolic murmur that extends into diastole.
Depending on the system affected by hypoperfusion, patients can also present with:
- Respiratory failure
- Cardiac hypertrophy
- Renal dysfunction
- Inability to tolerate feeding
- Necrotizing enterocolitis
Adult presentation at physical exam
Among adults, closure is indicated for patients with:
- Left atrial and/or LV enlargement
- Pulmonary arterial hypertension
- Left-to-right shunt
- Prior endarteritis
- Diagnostic evaluation
Echocardiography and/or angiography can differentiate between PDA and coronary arteriovenous fistulas, which can have a similar presentation.
- PDA treatment
The American College of Cardiology noted that many PDAs are now closed in infancy or childhood with catheter-based or surgical approaches. For those whose ductus remains patent in adulthood, catheter- based or surgical intervention consideration depends on the symptoms and physiological expression of the lesion.1
Yet in clinical practice there are inconsistencies in therapy strategies, especially for small and hemodynamically insignificant PDA. Some clinicians support closure to eliminate the lifelong risk of infective endarteritis, and others maintain that it is unnecessary.11
- When transcatheter PDA closure is performed
When an infant is asymptomatic or well controlled on medical therapy, closure treatment may be delayed until transcatheter therapy can be offered.
Adults with PDA are better suited for percutaneous closure due to high rates of success and low rates of complications.
Even when patients present with a small asymptomatic PDA, transcatheter device closure is a reasonable therapeutic approach.1
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional advice. Consult with a physician or qualified healthcare provider for appropriate medical advice.
LITTLE HEROES NEED OUR SUPPORT
See how Abbott's Little Heroes initiative provides life-changing technologies and support for children that do not have access to healthcare.
MAT-2415070 v2.0 | Item approved for U.S. use only.
