Epic™ Plus Mitral And Aortic Stented Tissue Valves
The latest generation of the Epic™ Platform continues the Epic product family’s history of excellence. The future-forward design of the Epic Plus Mitral and Aortic Valves gives you and your patients more choices when it comes to preserving long-term treatment paths.
Epic™ Plus Mitral And Aortic Stented Tissue Valves
The latest generation of the Epic™ Platform continues the Epic product family’s history of excellence. The future-forward design of the Epic Plus Mitral and Aortic Valves gives you and your patients more choices when it comes to preserving long-term treatment paths.
MADE FOR WHAT’S AHEAD
Built on the proven legacy of its predecessors, the Epic™ Plus Valve delivers everything you’ve come to expect from the Epic valve — plus significant improvements to radiopacity and implantability to enable future interventions and implantation for even the most challenging patient anatomies.
Mitral
- Low stent post height is less likely to obstruct the left ventricular outflow tract (LVOT)1
- Epic Plus Valve leaflets are not prone to forming a curtain between stent posts when opened; curtaining can result in LVOT obstruction
Aortic
- Can withstand up to approximately 8 atm pressure during balloon valvuloplasty procedures3
- Internally mounted leaflets and low stent post height reduce coronary obstruction4
- Increased visibility at valve annulus and stent posts under fluoroscopy for transcatheter aortic valve replacement (TAVR) procedures

*For 27 mm valve size, measured from the farthest point of the cuff.
- Streamlined customizable implantation
- Stent symmetry eliminates the need for specific orientation2
- Flexible polymer stent offers more forgiving insertion and seating
- Short rinse time of 2 x 10 seconds2
- Epic Plus Mitral Valve ratcheting holder reduces risk of suture looping and offers a true one-cut release
- Epic Plus Platform Cuff Options allow for secure suture placement while limiting suture drag and parachuting forces
- Exceptional durability across patient populations
10 Years
97.3 ± 0.5%†
Freedom from
surgery for SVD,
all patient ages
(Epic Aortic Valve)595.7 ± 2.6%†
Freedom from
surgery for SVD,
all patient ages
(Epic Mitral Valve)598.6 ± 0.8%†
Freedom from
surgery for SVD,
all patient ages
(Epic Aortic and
Epic Mitral Valve)5Linx™ Anticalcification (AC) treatment may improve long-term performance and durability.*
*There is no clinical data currently available that evaluates the long-term impact of anticalcification tissue treatment in humans.
†From stand-alone aortic or mitral valve replacements.
PATIENTS WHO BENEFIT
Surgical valve treatment for mitral and aortic valve disease
The Epic™ Plus Mitral Stented Tissue Valve is indicated for patients requiring replacement of a diseased, damaged, or malfunctioning native mitral heart valve. It may also be used to replace a previously implanted prosthetic mitral valve.
While mitral valve repair is typically the treatment of choice to treat mitral valve dysfunction, it is not always feasible.
The Epic™ Plus Supra Stented Tissue Valve is indicated for patients requiring replacement of a diseased, damaged, or malfunctioning native aortic heart valve. It may also be used to replace a previously implanted prosthetic aortic valve.
- Considerations when selecting bioprosthestic valve candidates6
A bioprosthetic mitral or aortic valve may be a recommended option for patients under the following AHA/ACC treatment guidelines6:
- Patients with a contraindication for anticoagulation therapy or an anticipated nonadherence or inability to regulate vitamin K antagonists
- Patients over 70 years of age
- For patients 50—65 years of age, there is no clear consensus on choosing a mechanical vs. a tissue valve; newer-generation tissue prostheses may show greater freedom from structural deterioration, often for older patients
Mitral valve replacement
When repair is not feasible
- Symptomatic patients with severe primary MR (Stage D)6
- Asymptomatic patients with severe primary MR and LV systolic dysfunction (LVEF ≤ 60%, LVESD ≥ 40 mm) (Stage C2)6
- Advanced endocarditis7
Aortic valve replacement
- Symptomatic adults with severe, high-gradient AS (Stage D1)6
- Asymptomatic patients with severe AS (Stage C) and either an LVEF < 50% or who are undergoing cardiac surgery for other indications6
- Symptomatic patients with low-flow, low-gradient severe AS with reduced LVEF (Stage D2)6
CLINIC FINDER
KEY FEATURES
The platform for possibility
The Epic Platform has been proven over time and defined by strong hemodynamics, intuitive implantability, a future-forward design and more. The platform boasts the low ventricular protrusion in all valve sizes, a flexible polymer stent, individually selected porcine leaflets to ensure optimal coaptation and a flexible sewing cuff designed to minimize both in-implant suture drag and post-implant paravalvular leak.
- Epic™ Plus Supra Aortic Stented Tissue Valve features
- Silicone-Filled Cuff
- FlexFitTM Polymer Stent
- Unique Pericardial Shield
- Flexible Cuff
- Low Stent Post Height
Allows for supra-annular implantation
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
Mitigates PVL and easily fits patient anatomy
Mitigates risk of coronary obstruction
EPIC™ SUPRA AORTIC STENTED TISSUE VALVE FEATURES
 12345
12345SILICONE-FILLED CUFF
Allows for supra-annular implantation
FLEXFIT™ POLYMER STENT
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
UNIQUE PERICARDIAL SHIELD
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
FLEXIBLE CUFF
Mitigates PVL and easily fits patient anatomy
LOW STENT POST HEIGHT
Mitigates risk of coronary obstruction
- Epic™ Plus Mitral Stented Tissue Valve features
- Optimal Leaflet Design and Matching
- Low Ventricular Protrusion
- FlexFitTM Polymer Stent
- Unique Pericardial Shield
- Flexible Cuff
- Ratcheting Holder
Reduces regurgitation risk
Avoids LVOT obstruction
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
Mitigates PVL and easily fits patient anatomy
Streamlines implant, reduces risk of suture looping, and offers true one cut holder release
EPIC™ MITRAL STENTED TISSUE VALVE FEATURES
 123456
123456OPTIMAL LEAFLET DESIGN AND MATCHING
Reduces regurgitation risk
LOW VENTRICULAR PROTRUSION
Avoids LVOT obstruction
FLEXFIT™ POLYMER STENT
- Withstands approximately 8 atm pressure during balloon valvuloplasty procedures1
- Eases implant
- Accommodates MIS procedures.
UNIQUE PERICARDIAL SHIELD
Provides a tissue-to-tissue interface to help prevent the risk of abrasion
FLEXIBLE CUFF
Mitigates PVL and easily fits patient anatomy
RATCHETING HOLDER
Streamlines implant, reduces risk of suture looping, and offers true one cut holder release
- Low profile design for now and later
Curtaining is a characteristic of bioprosthetic valves in which the leaflets, when opened, stand tall or form a “curtain” between stent posts.8,* Epic/Epic Plus Mitral leaflets are not prone to curtaining, which can result in less LVOT obstruction.8,*,†
Leaflet behavior when opened
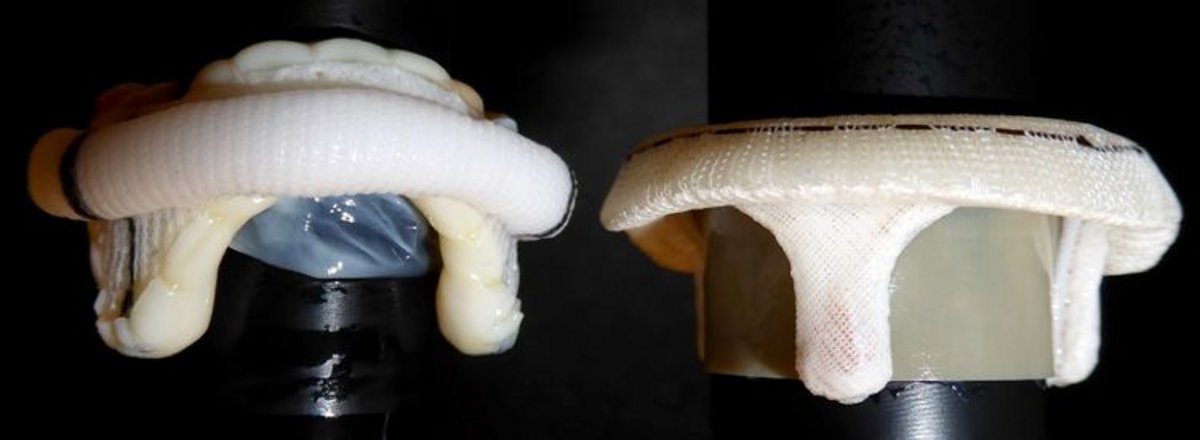
25 mm Epic Mitral† valve (left) and Magna Mitral Ease‡ (right), with representative rods inserted *Results based on internal testing.
†The Epic and Epic Plus Valves are designed to the same specifications for dimension and tissue behavior.
‡Indicates a third-party trademark, which is property of its respective owner. - Preserving the LVOT
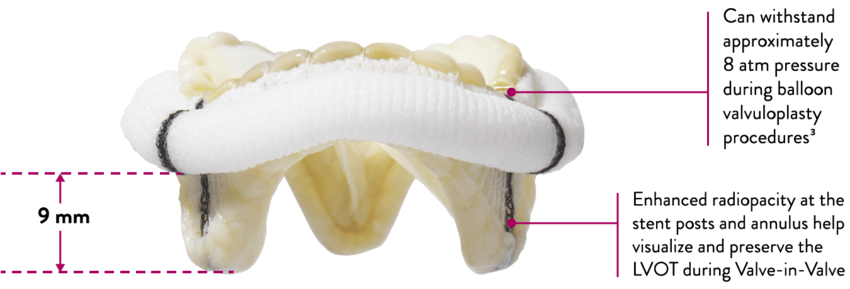

*For 27 mm valve size, measured from the farthest point of the cuff.
†Indicates a third-party trademark, which is property of its respective owner. - Anticalcification advantages
Abbott’s unique Linx AC technology—to promote anticalcification*—is designed to improve long-term performance and valve durability. The Linx AC treatment has demonstrated resistance to calcification by:
- Extracting lipids11
- Reducing free aldehydes12,13
- Minimizing cholesterol uptake14
- Stabilizing leaflet collagen14
*No clinical data currently available have evaluated the long-term impact of anticalcification tissue treatment in humans.
MAT-2001799 v9.0 | Item approved for U.S. use only.