NAVITOR™ TAVI SYSTEM
Stable delivery. Remarkable performance. Future ready.
EXCELLENT JUST GOT BETTER
Your work demands a safe, intuitive and future ready TAVI system that achieves excellent outcomes, every time. Our Navitor™ TAVI System is intuitive, precise and accurate — with excellent hemodynamics and durability. There are Navitor Vision* Valve sizes for a wide range of anatomies.
30-day outcomes1:
0%
MODERATE OR SEVERE PVL
1.9%
ALL-CAUSE
MORTALITY
1.9%
DISABLING
STROKE
4.2%
MAJOR VASCULAR
COMPLICATIONS
7.4
mm Hg
MEAN
GRADIENT
PATIENTS WHO BENEFIT
The Navitor TAVI System offers a lifesaving treatment option for individuals with symptomatic severe aortic stenosis who are at high or greater risk for traditional open-heart surgery. This includes patients with considerations related to age, frailty, or other health conditions, making TAVI a viable and less invasive option.
NAVITOR VISION VALVE
Attention to every detail
The Navitor Vision Valve is engineered with meticulous attention to detail, ensuring optimal outcomes for TAVI procedures.
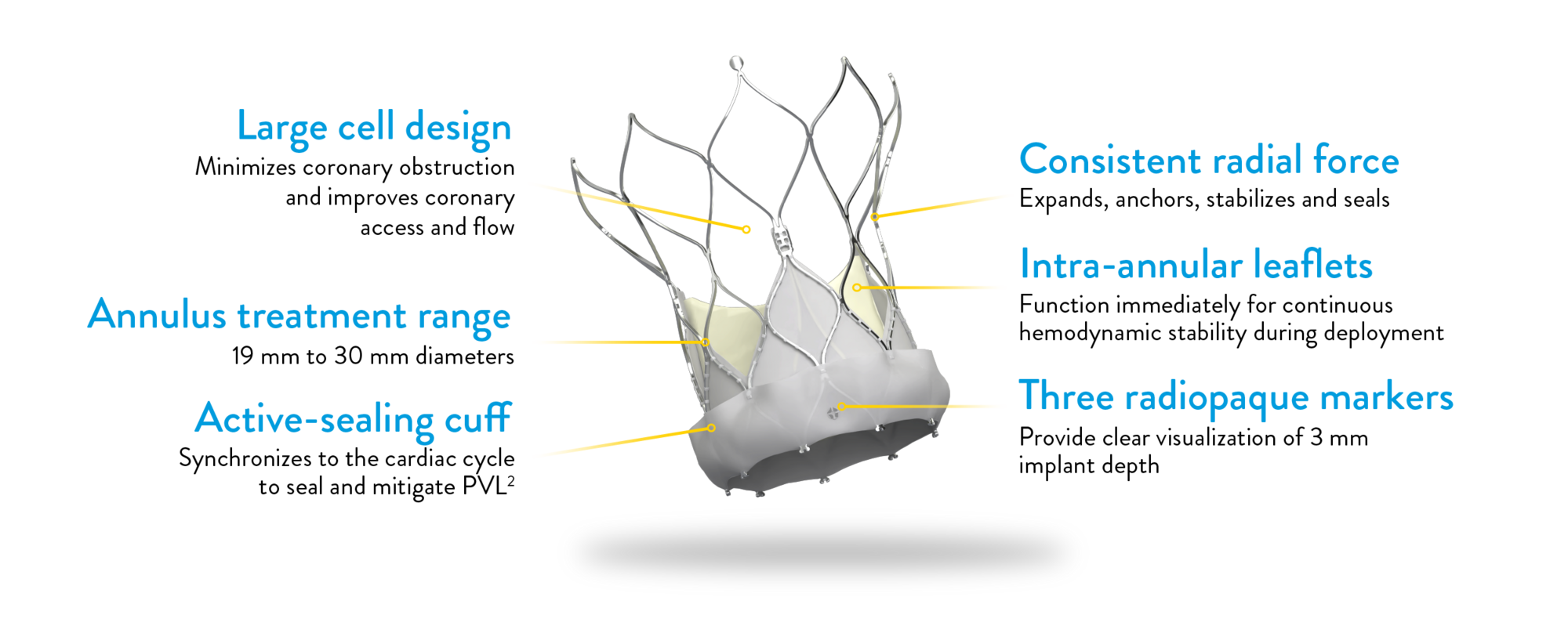
*Labeled as Navitor and Navitor Titan with Vision Technology
FLEXNAV™ DELIVERY SYSTEM:
THE CONFIDENCE OF CONTROLLED DEPLOYMENT
Low-profile, highly flexible catheter enables excellent access and deliverability.

**14 F and 15 F equivalent
CONNECT WITH YOUR LOCAL REPRESENTATIVE TODAY
SEE THE LATEST CLINICAL
DATA FOR NAVITOR
MAT-2214014 v5.0 | Item approved for U.S. use only.