MECHANICAL HEART VALVES
Unique design features have established Abbott mechanical heart valves—including the Regent™ and Masters Series—as a gold standard for reliability and performance, while maintaining low complication rates over the long term.
MECHANICAL HEART VALVES
Unique design features have established Abbott mechanical heart valves—including the Regent™ and Masters Series—as a gold standard for reliability and performance, while maintaining low complication rates over the long term.
CONFIDENTLY IMPLANT THE MOST TRUSTED AORTIC AND MITRAL MECHANICAL VALVES IN THE WORLD
- Design-driven hemodynamic confidence & low thrombogenicity
Abbott mechanical heart valves lead the way with a proven bileaflet design that results in low thrombogenicity and excellent patient outcomes.
- Leaflets open to an 85-degree angle in systole due to their upstream positioning, enabled by unique pivot guards (shown at right)
- Strong, uniform velocities within hinge recesses aid washout of blood elements3
- Low carbon surface area means less thrombus formation
- Orifice-to-annulus ratio (as high as 84%) ensures large effective orifice areas (EOAs) and reduces prosthesis-patient mismatch4
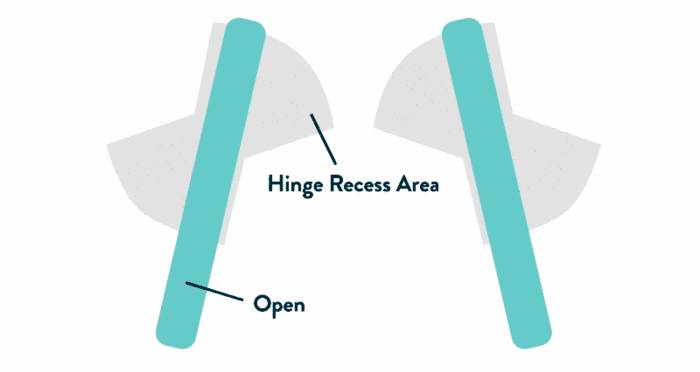
- Unique design features
The Pivot Guard Design
An Abbott HallmarkFeatured in all Abbott Mechanical Heart Valves, the Pivot Guard offers benefits both during implant and post-implant
- Recessed design minimizes interaction with sub-annular native mitral valve apparatus
- Minimized carbon surface area can lessen thrombus formation
- Pivot guards help shield pivot mechanism from pannus ingrowth
- Enables a consistent 85-degree opening angle, promoting minimized leaflet flutter and more laminar flow through the orifice5

Low Thrombogenicity
A necessity in MHV replacementsDesigned with low thrombogenicity at the forefront
- Large internal diameters up to 26.1 mm available promote low pressure gradients6
- Low gradients can help minimize shear stress and thrombogenicity7
- Uniform velocities within hinge recesses help aid washout of blood elements3
- Abbott hinge design enables complete leaflet sweep, further aiding washout
- Abbott hinge design enables complete leaflet sweep, further aiding washout
PATIENTS WHO BENEFIT
Excellent function and durability throughout a patient’s lifetime
Although the prevalence of mitral valve disease is 2 to 3 times higher than aortic valve disease, aortic valve surgeries (excluding transcatheter procedures) are performed 1.6 times more commonly than mitral valve surgeries. The undertreatment of mitral valve disease may be related to the slower progression of symptoms compared with aortic disease, as well as a potential lack of adherence to guidelines for intervention.8
- Aortic Stenosis (AS) AHA/ACC Treatment Guidelines
Severe AS presents with exertional dyspnea, syncope, angina, and progression to heart failure. Early intervention produces a better outcome in preventing the clinical deterioration of AS.2 Severe AS affects 3.4% of the population over 60 years of age.9
Surgical aortic valve replacement (SAVR) is recommended for symptomatic patients with severe AS (Stage D) and asymptomatic patients with severe AS (Stage C) who meet an indication for AVR when surgical risk is low or intermediate. Outcomes after SAVR are excellent in patients who do not have a high procedural risk, resulting in improved survival rates, reduced symptoms, and improved exercise capacity.10
Choosing a Regent™ Mechanical Heart Valve
The primary advantages of a Regent™ mechanical valve include durability, since mechanical valves are designed to last a lifetime.8 Mechanical valves also avoid the potential of eventual calcification, which can occur with tissue valves. Furthermore, the excellent hemodynamics of the Regent aortic valve is another advantage to consider.
However, any mechanical valve confers a thrombogenic potential which could eventually result in embolic stroke. This is mitigated by the requirement that patients adhere to lifelong anticoagulants, such as warfarin, to preclude thrombus formation.
Bleak prognosis without treatment
Mortality rates are approximately 25% at 1 year and 50% at 2 years in symptomatic AS patients on medical therapy who do not undergo AVR.11
- Mitral Regurgitation (MR) AHA/ACC Treatment Guidelines
MR is the most common valve disease worldwide.5 Intervention for patients with primary MR consists of either surgical mitral valve repair or replacement, with repair preferred over replacement if a successful and durable repair can be achieved. Mitral valve surgery is recommended in10:
- Symptomatic patients with chronic, severe primary MR (stage D) and left ventricular ejection fraction (LVEF) > 30%
- Asymptomatic patients with chronic, severe primary MR and LVEF 30%–60% and/or LV end-systolic dimension ≥ 40 mm (Stage C2)
- Chronic, severe primary MR patients undergoing cardiac surgery for other indications
- Patients with chronic, severe secondary MR (stages C and D) at the time of other cardiac surgery
Choosing the Masters Series Mitral Mechanical Heart Valve
The Masters Series mitral mechanical heart valve is intended for use as a replacement valve in patients with a diseased, damaged, or malfunctioning mitral valve. The device may also be used to replace a previously implanted mitral prosthetic valve.
The primary advantages of a Masters Series mitral mechanical valve include durability, since mechanical valves are designed to last a lifetime.13 Mechanical valves also avoid the potential of eventual calcification, which can occur with tissue valves. The excellent hemodynamics and low thrombogenicity of the Masters Series mitral valve are other advantages to consider.
However, mechanical valves confer a thrombogenic potential and require long-term anticoagulant therapy to preclude thrombus formation. Ultimately, the choice of mechanical versus bioprosthetic valve replacement for all patients, but especially for those between 50 and 70 years of age, must account for the trade-offs between durability (and the need for reintervention), bleeding, and thromboembolism.10
Late referral for surgical intervention is associated with reduced survival
There remains an important and substantial opportunity to decrease long-term mortality in patients with mitral valve disease by following established guidelines and encouraging earlier referral for intervention.8
Addressing an unmet need for high-risk pediatric patients
Only Abbott provides the world's smallest pediatric mechanical heart valve, the 15 mm Masters HP, for aortic and mitral heart valves. Developed to address an unmet need for high-risk pediatric patients who formerly had limited options. The patients who need the valve are usually younger than 1 year old with complex heart defects, have had multiple prior heart surgeries and require a valve replacement.
REGENT KEY FEATURES
Regent gives you implantability options
LOW VALVE IMPLANT HEIGHT HELPS ENSURE CORONARY OSTIA CLEARANCE

REPLICA SIZING WITH THE 907 SIZER GIVES YOU CONFIDENCE THAT YOU ARE USING THE MOST APPROPRIATELY SIZED VALVE
Two distinct cuff types for the best fit for your patients

FLEXCUFF™

FLEXCUFF™

STANDARD CUFF

STANDARD CUFF
- The regent aortic valve features exceptional hemodynamics in small aortic root patients15,16,*
.
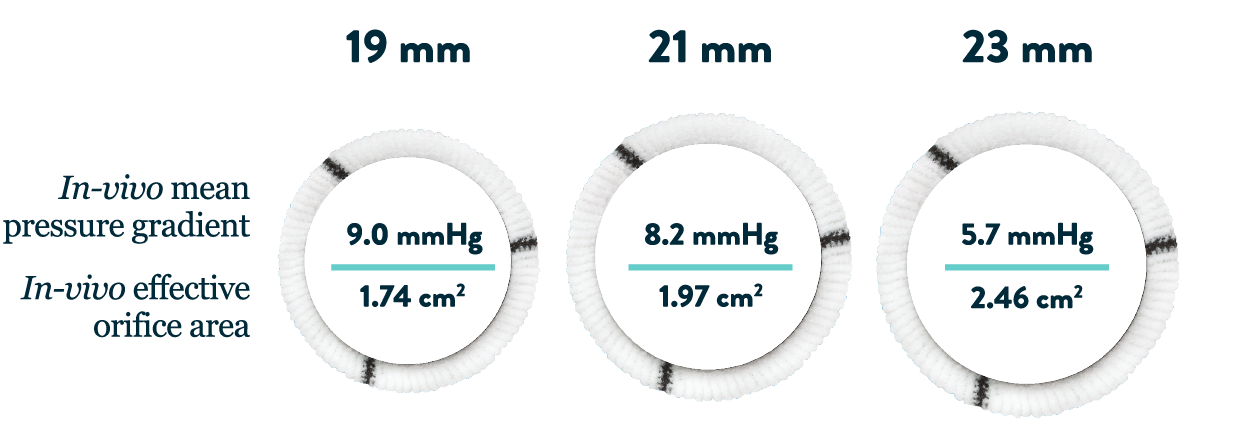
*Measured one-year post-surgery.

MASTERS SERIES KEY FEATURES
Implantability options with Masters Series
The Masters Series Mechanical Heart Valves are rotatable, bileaflet valves designed for secure implantation. The portfolio includes an expansive range of sizes to help you make the best decisions for your patients and the Hemodynamic Plus™ (HP) models are designed to provide a larger effective orifice area.
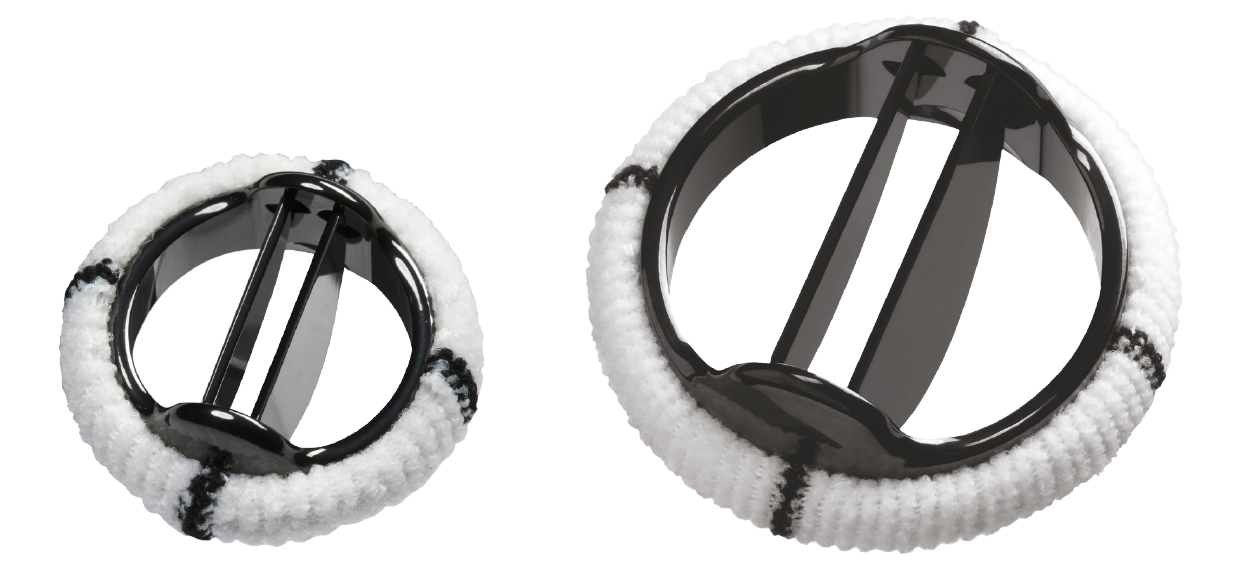
The 15MM pediatric heart valve joins the family of
17–27MM Masters HP mechanical heart valves and is suitable for treating newborns and babies
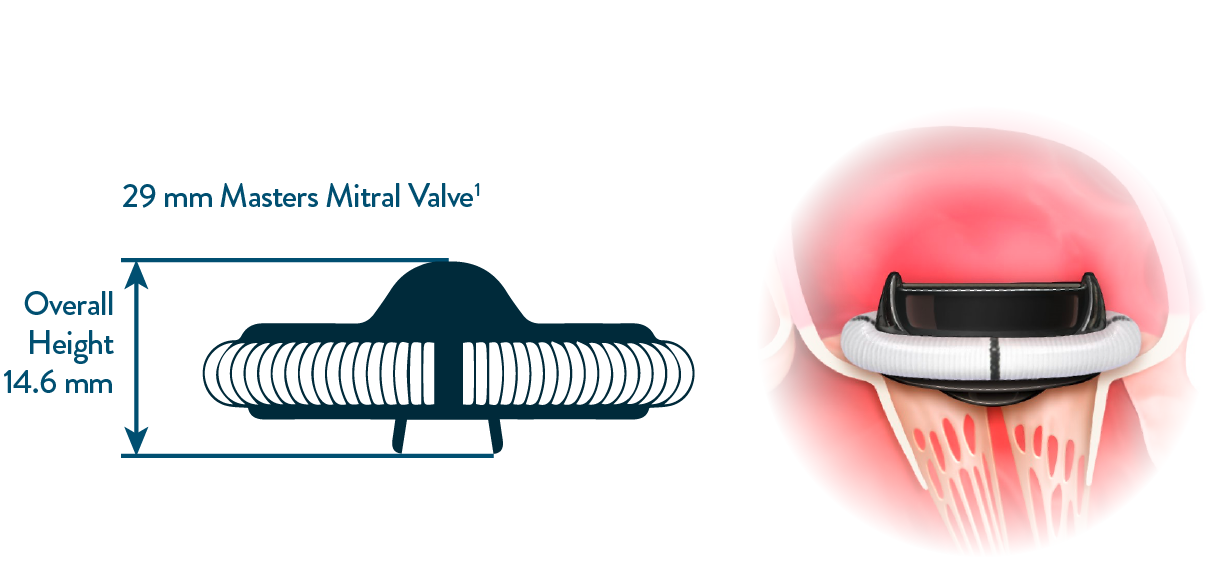
Low valve implant height minimizes interference with subvalvular
structures in the mitral position
Two distinct cuff types for the best fit for your patients

EXPANDED CUFF

EXPANDED CUFF

STANDARD CUFF

STANDARD CUFF
CLINICAL DATA
See the latest clinical data for Regent and Masters Series.
MAT-2010286 v8.0 | Item approved for U.S. use only.




