BETTER EVIDENCE TO BUILD CONFIDENCE
Abbott provides a robust body of clinical evidence encompassing clinical data and outcomes from tens of thousands of patients across our structural heart portfolio of products.*
IN-DEPTH DATA ABOUT STRUCTURAL HEART PRODUCTS
With our Clinical Insights and Bulletins you can take a deeper dive into clinical outcomes related to structural heart products. Designed for ease of viewing, these pieces give you a broader perspective about study outcomes along with product safety and performance.
Transcatheter Valve Solutions
TAVI
Structural Interventions

Amplatzer™ Amulet™ LAA Occluder
Read moreClinical Experience with Amplatzer™ Amulet™ Left Atrial Appendage Occluder for Reducing Risk of Ischemic Stroke in Patients with AF.
Surgical Valve Solutions

Masters Series Mitral Mechanical Heart Valves
Read moreKey Mitral MHV Selection Considerations: Durability,
Size Range, Hemodynamics, and Implantability1
Epic™ Mitral Stented Tissue Valve with Linx™ AC Technology
Read moreLong-Term Outcomes and Freedom from Calcification

Epic™ Mitral And Epic™ Supra Stented Tissue Valves
Read moreThree-Year Outcomes of Valve-in-Valve Intervention within the Epic™ Supra and Epic™ Mitral Valves in a Medicare Population

Epic™ Supra Aortic Stented Tissue Valve With Linx™ AC Technology
Read moreTen-Year Outcomes of a Contemporary Supra-annular Porcine Aortic Bioprosthesis in a Medicare Population1

Epic™ Mitral Stented Tissue Valve With Linx™ AC Technology
Read moreEpic Mitral – Ten Year Clinical Outcomes of SMVR in a Medicare Population1
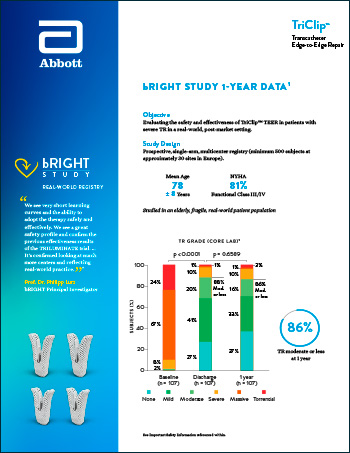
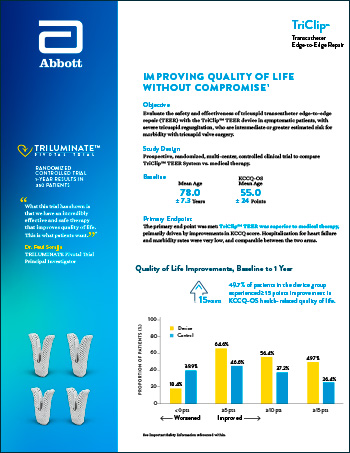
CLINICAL DATA | TEER SOLUTIONS
CLINICAL DATA | SURGICAL VALVE SOLUTIONS
CLINICAL DATA | STRUCTURAL INTERVENTIONS
MAT-2116292 v17.0 | Item approved for U.S. use only.