AMPLATZER™ TALISMAN™
PFO OCCLUDER CLINICAL DATA
Effective PFO closure is made easier with the Amplatzer™ Talisman™ PFO Occluder.5 For patients who have experienced a PFO-associated stroke, clinical trials have shown that they can benefit from PFO closure.1-3 This minimally-invasive procedure significantly reduces the risk of recurrent ischemic stroke and offers an excellent safety profile.
THE LANDMARK RESPECT TRIAL1
Still the definitive clinical trial on PFO closure
Study design
The RESPECT trial is the largest trial ever conducted on PFO closure. This trial studied the Amplatzer™ PFO Occluder, on which the new Amplatzer™ Talisman™ device is based.
It stands apart from other PFO closure studies since it's the largest clinical trial with the most extensive follow-up, spanning 13 years. The 980 enrolled patients were followed for a median of 5.9 years. Researchers ultimately collected 5,810 patient-years of data—almost 2 times more than other PFO trials.
The trial was conducted at 69 centers across the U.S. and Canada and included patients on anticoagulation therapy – a real-world cross-section of patients – unlike other PFO trials, REDUCE2 and CLOSE3.
The findings
The RESPECT trial offers conclusive evidence that using the Amplatzer PFO Occluder to close the PFO in patients with a PFO-associated stroke is more beneficial than medical therapy alone, in reducing the risk of another stroke.
Summary of findings
Excellent procedural results
The RESPECT trial data revealed high rates of technical and procedural success, and excellent closure.1 At only 6 months, most patients also demonstrated freedom from shunt — both at rest and during Valsalva.1
99.1%
TECHNICAL SUCCESS*
96.1%
PROCEDURAL SUCCESS†
94.2%
EFFECTIVE CLOSURE
(N ≤ 9 BUBBLES)
Excellent safety1
0%
DEVICE EMBOLIZATION
0%
DEVICE EROSION
0%
DEVICE THROMBUS
Note: Rates are calculated based on data in final publication.
*Successful delivery and release of the device at the time of first procedure for subjects in whom a study device was attempted.
†Successful implant at the time of the first procedure with no reported in-hospital serious adverse events.
- Significantly lower risk of recurrent stroke
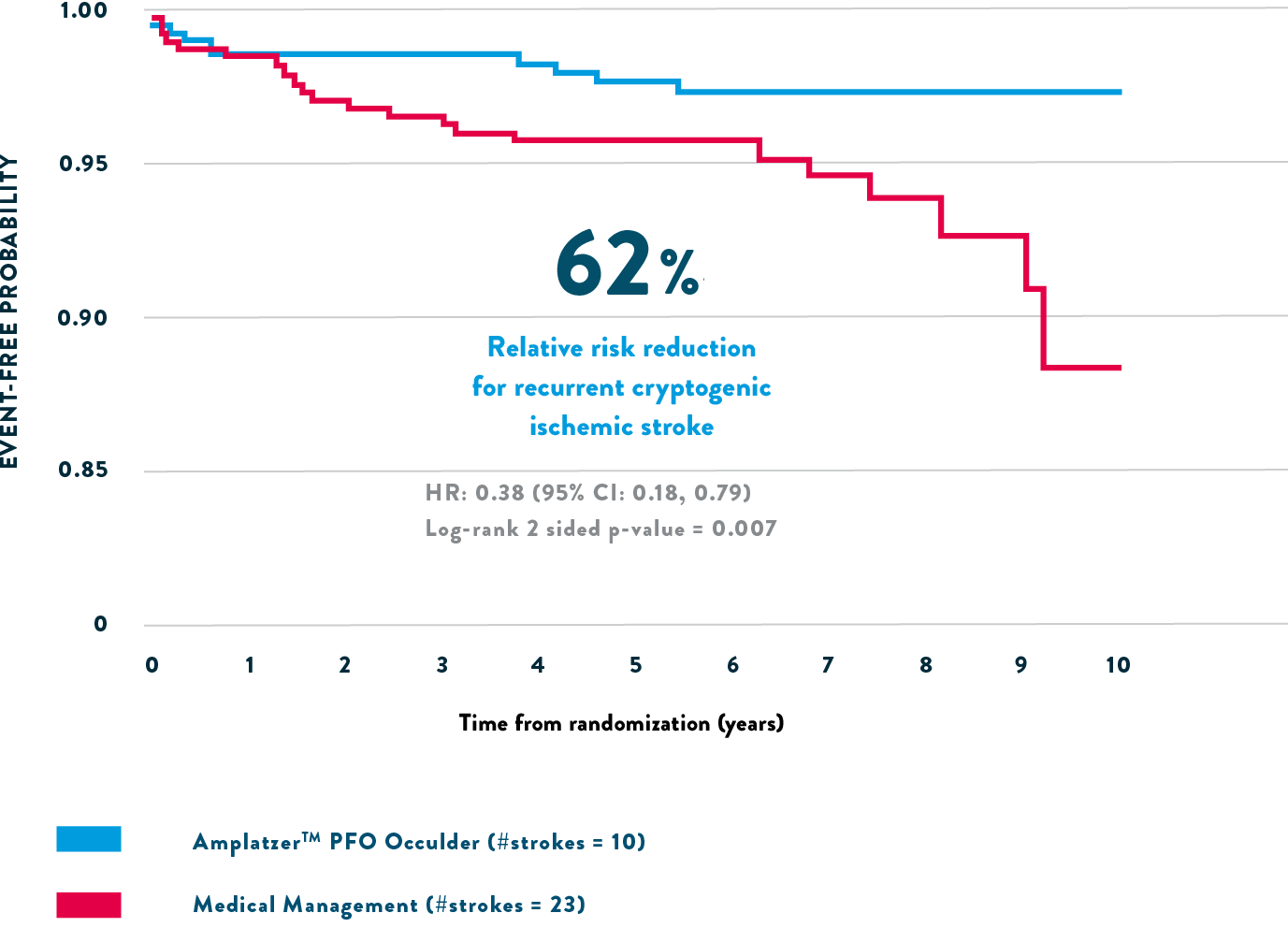
The RESPECT trial revealed a 62% relative risk reduction for recurrent cryptogenic ischemic stroke with the Amplatzer™ PFO Occluder vs medical therapy.1
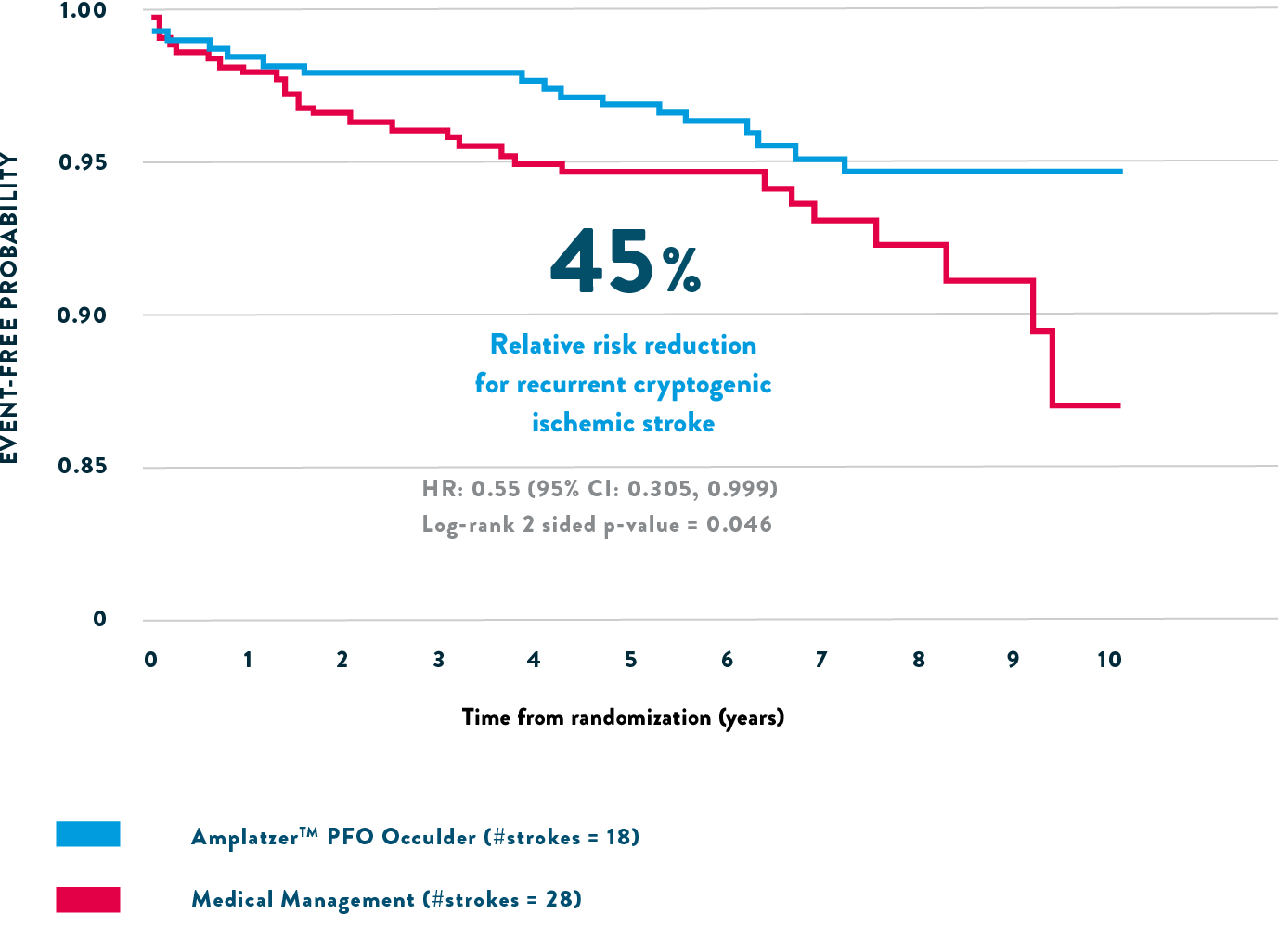
The trial showed there was a 45% relative risk reduction for any recurrent ischemic stroke over nearly 6 years of follow-up, compared to medical therapy.1
- Low rates of atrial fibrillation
The RESPECT trial also revealed low rates of serious atrial fibrillation (AF) with the Amplatzer™ device, consistent with medical therapy.1
MAT-2500547 v1.0 | Item approved for U.S. use only.

